
All three valence electrons and two lone pair electrons occupy the sp 3 hybrid orbital in amines, whereas three valence electrons lie in almost pure p orbitals and lone pair electrons occupy the remaining s orbital in bismuthines. Lu Hf Ta W Re Os Ir Pt Au Hg (La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb. These electrons are arranged according to specific rules of. The lack of basicity is because of the high s-character of the lone pair electron. five 3d orbitals), one electron will enter each of the available orbitals. The total number of electrons in bromine is thirty-five. We now have a choice of filling one of the 2p orbitals and pairing the electrons or of leaving the electrons unpaired in two different, but degenerate, p orbitals. The remaining two electrons occupy the 2p subshell. Four of them fill the 1s and 2s orbitals. the halogen with electrons in the 6 p atomic orbitals. Carbon (atomic number 6) has six electrons. from the atomic 6s populations of the molecular orbitals on the gold ion. The electronic configuration of atoms follows a standard. The total electron density at the nucleus p(0) depends on the nature of the.
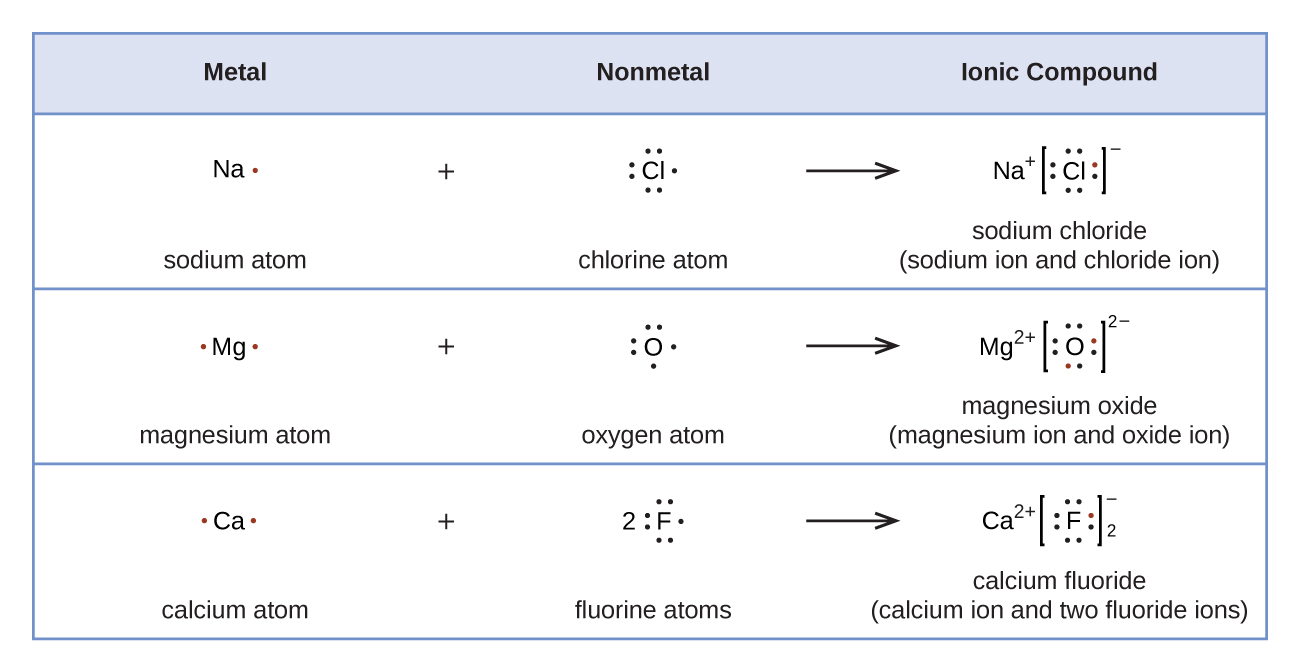
an element with three unpaired 5 d electrons. Atomic orbitals define the distribution of electrons in an elements electron configuration. An important outgrowth of this electronic configuration of bismuth is that trivalent bismuthines exhibit little basicity. Using only the periodic table inside the front cover of the text, write the expected ground-state electron configurations for a. Of all group-15 elements, the heavy bismuth atom has the lowest tendency to form a hybrid orbital. Through the next nine elements, in increasing order of atomic number, electrons are added to the 3d orbitals until, at the element zinc, they are entirely. creates bonds from overlap of atomic orbitals ( s, p, d ) and hybrid orbitals ( sp, sp2, sp3 ) combines atomic orbitals to form molecular orbitals (,, , ) forms or bonds. In this article, we discuss the implementation of the velocity-gauge RT-TDDFT equations for electron dynamics within a linear combination of atomic orbitals. considers electrons delocalized throughout the entire molecule. In general, these structures are determined by the combination of electronic and steric requirements. considers bonds as localized between one pair of atoms.
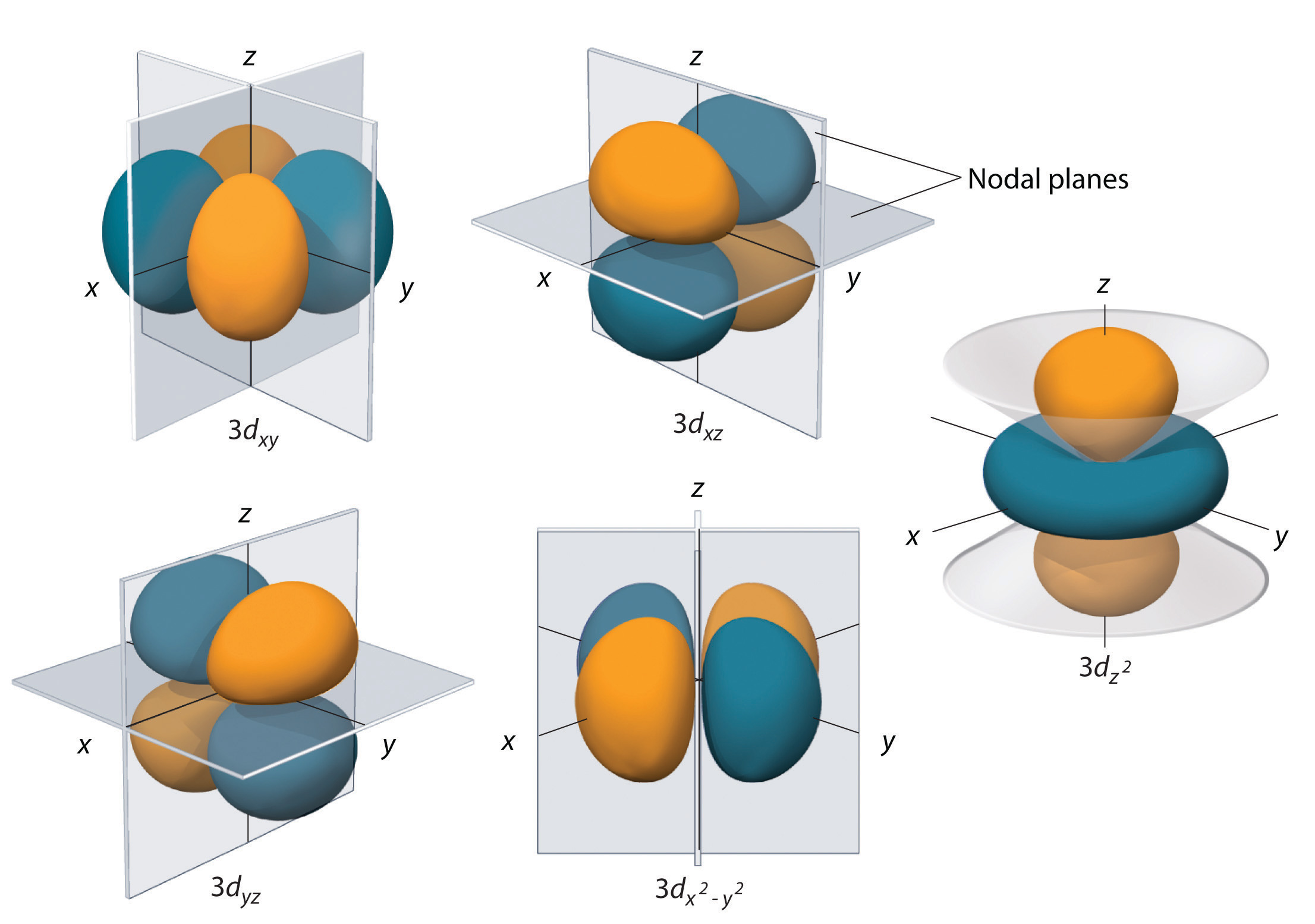
Going down the periodic table, the geometry of pnictogenides is gradually distorted from the ideal tetrahedral structure and thus, the bond angles of the C-Bi-C bonds in bismuthines are nearly 90°, which are almost the same as those of 6p orbitals. Simple amines have mostly an ideal tetrahedral structure because of the almost ideal sp 3 hybrid orbitals that the central nitrogen atoms have. Some relevant inorganic bismuth compounds are included, which may be of interest to organic chemists, but transition metal complexes are not included. The criterion for compounds to be included in this chapter is not strictly limited to “organic” bismuth compounds.


 0 kommentar(er)
0 kommentar(er)
